

Seemingly, there is a chasm between numerical and physical resolutions of the covalent bond. While bond formation is arguably the most fundamental chemical process, its physical origin is still the subject of debate, even today when accurate quantitative molecular electronic structure calculations of ever-increasing accuracy and complexity have become widely available. In order to resolve this impasse we show that quantum mechanics affords us a complementary dynamical perspective on the bonding mechanism, which agrees with that of Hellmann and Ruedenberg, while providing a direct and unifying view of atomic reactivity, molecule formation and the basic role of the kinetic energy, as well as the important but secondary role of electrostatics, in covalent bonding.Ĭovalent chemical bonding is undoubtedly a central concept in Chemistry. We and many others have helped verify the validity of the Hellmann–Ruedenberg proposal that a decrease in kinetic energy associated with interatomic delocalization of electron motion is the key to covalent bonding but contrary views, confusion or lack of understanding still abound. In particular, we focus on the bonding analysis of Hellmann (1933) that was brought into modern form by Ruedenberg (from 1962 on). We summarize the mechanistic bonding models and the debate over the last 100 years, with specific applications to the simplest molecules: H 2 + and H 2.
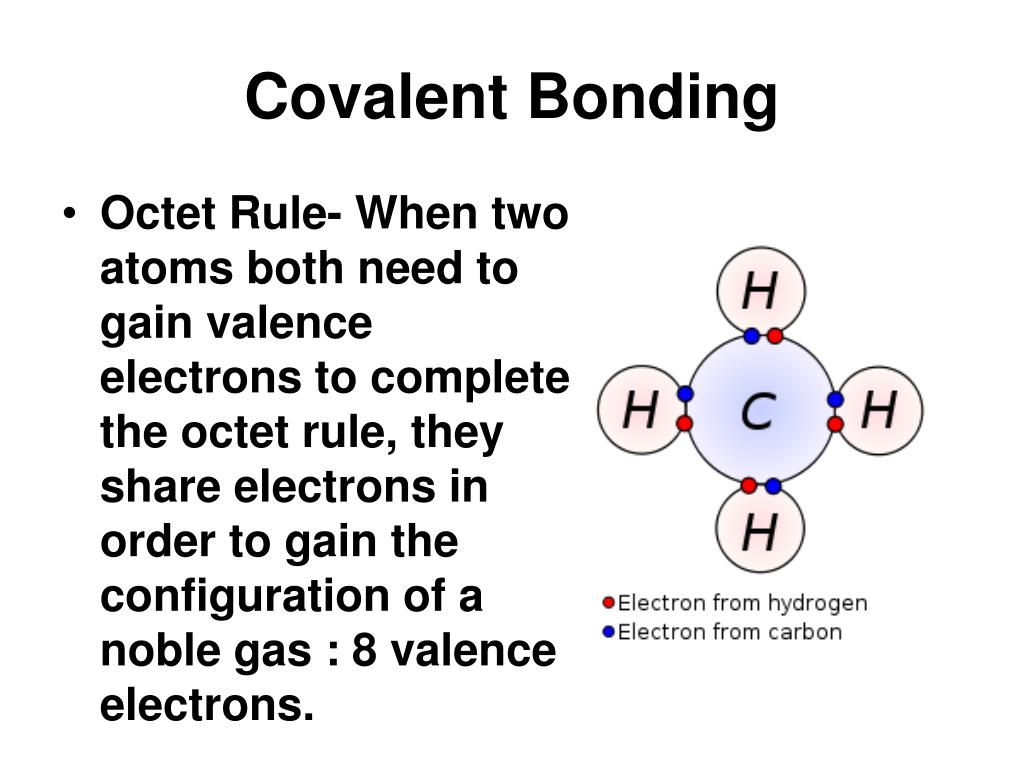

Our aim here is to unify two seemingly different explanations: one in terms of energy, the other dynamics. We address the paradoxical fact that the concept of a covalent bond, a cornerstone of chemistry which is well resolved computationally by the methods of quantum chemistry, is still the subject of debate, disagreement, and ignorance with respect to its physical origin.


 0 kommentar(er)
0 kommentar(er)
